Insulin
Englisch: insulin
Definition
Das Peptidhormon Insulin reguliert die Aufnahme von Glucose in Körperzellen. Es wirkt blutzuckersenkend und spielt eine wesentliche Rolle bei der Entstehung und Therapie des Diabetes mellitus. Insulin ist der natürliche Gegenspieler des Hormons Glucagon.
Der Name Insulin stammt vom lateinischen Wort "insula" (Insel) ab, da das Hormon in den Inselzellen der Bauchspeicheldrüse (Pankreas) gebildet wird.
Geschichte
In Jahr 1921 gelang es Frederick Banting und Charles Best an der Universität in Toronto erstmals, Insulin aus Pankreasgewebe zu gewinnen. Schon seit einigen Jahrzehnten war klar, dass Sekrete der Bauchspeicheldrüse den Blutzuckerspiegel senken können. Frühere Versuche anderer Wissenschaftler waren jedoch nicht erfolgreich, da sie die komplette gemahlene Bauchspeicheldrüse verwendeten, wobei andere Verdauungssäfte des Pankreas das Insulin zerstörten. Die ersten Versuche von Banting und Best wurden an Hunden durchgeführt, denen die Bauchspeicheldrüse operativ entfernt worden war. 1922 gelang ihnen die erste Rettung eines 13 Jahre alten Diabetikers, Thomas Leonhard, der seit anderthalb Jahren an der Krankheit litt und bereits ins Koma gefallen war. 1923 wurde erstes tierisches Insulin aus Rindern und Schweinen eingesetzt und im selben Jahr erhielten Banting und John MacLeod, der Leiter des Instituts, den Nobelpreis für Physiologie oder Medizin. Sie teilten den Preis freiwillig mit Best und James Collip, der einen verbesserten Extrakt erfunden hatte.
In den folgenden Jahrzehnten wurde Insulin aus den Bauchspeicheldrüsen von Rindern und Schweinen großtechnisch gewonnen. Rinder- und Schweineinsulin unterscheiden sich vom menschlichen Insulin nur geringfügig, beim Schwein ist nur eine, beim Rind sind drei Aminosäuren durch andere ersetzt. Obwohl auch tierisches Insulin beim Menschen wirkt, wurde trotzdem versucht, menschliches Insulin zu produzieren. 1946 kam NPH-Insulin (Neutrales-Protein-Hagedorn) zum Einsatz, das erste gewebsneutrale Insulin. 1978 gelang es erstmals, Humaninsulin durch gentechnisch veränderte Bakterien herzustellen, welches exakt dem menschlichen Insulin entspricht. Inzwischen übernehmen auch Hefepilze diese Aufgabe. Seit 1996 sind auch künstliche Insuline (Insulinanaloga) verfügbar, die schneller wirken als natürliches Insulin.
Biochemie
Struktur
Insulin ist ein Makromolekül mit einem Molekulargewicht von etwa 5.700 Dalton. Es besteht aus zwei längeren Polypeptiden, einer A-Kette mit 21 und einer B-Kette mit 30 Aminosäuren. Die beiden Ketten sind durch zwei Disulfidbrücken miteinander verbunden, die zwischen den Cystein-Bausteinen der Polypeptide ausgebildet werden. Eine dritte Disulfidbrücke besteht innerhalb der A-Kette. Sie dient der Stabilisierung der Raumstruktur.
Durch die Ausbildung von Wasserstoffbrückenbindungen zwischen den Sauerstoffatomen der Carbonylgruppen und den Wasserstoffatomen der Amidgruppen der beiden Polypeptidketten ensteht die Sekundärstruktur des Insulins: Die Molekülketten ziehen sich schraubenförmig zusammen. Die A-Kette bildet zwei α-Helices, die durch einen Aminosäurebaustein voneinander getrennt werden. Die B-Kette wickelt sich zu ca. 40 % zu einer α-Helix auf.
In Lösung hat Insulin die Tendenz, unter Ausbildung von Wasserstoffbrücken Dimere zu bilden, d.h. sich zu Molekülpaaren zusammenzulagern. In Anwesenheit von Zink bilden sich aus den Dimeren sogenannte Hexamere.
Biosynthese
Insulin wird in den sogenannten beta-Zellen der Langerhans-Inseln des Pankreas synthetisiert. Die genetische Information für die Synthese von Insulin wird von nur einem Gen-Lokus im kurzen Arm des Chromosom 11 kodiert. Das Gen besteht aus rund 300 Nukleotiden und enthält zwei Introns und drei Exons.
Die aus dem Gen transkribierte mRNA wird in den Ribosomen des rauen endoplasmatischen Retikulum (RER) zunächst in ein inaktives Präproinsulin übersetzt, ein Peptid aus 110 Aminosäuren. Es besteht aus einer Signalsequenz (leader), an die sich zunächst die 30 Aminosäuren der B-Kette schließen, danach ein C-Peptid (connecting peptide) und schließlich die A-Kette aus 21 Aminosäuren:
- Signalsequenz --- B-Kette --- C-Peptid --- A-Kette
Die Signalsequenz sorgt dafür, dass Präproinsulin in den Innenraum des endoplasmatischen Retikulums transportiert wird. Dort erfolgt die weitere "Reifung" des Peptidhormons: Durch Abspaltung der Signalsequenz und Bildung von drei Disulfidbrücken entsteht das Proinsulin (86 Aminosäuren). Im Verlauf der weiteren Reifung wird die C-Kette durch spezifische Peptidasen abgespalten. Das Insulinmolekül wird dann als Hexamer durch ein Zinkion stabilisiert in Vesikeln an der Zellmembran der beta-Zelle gespeichert.
Ausschüttung
Ein steigender Blutzuckerspiegel (ab ca. 4 mmol Glucose/l Blut) ist der wichtigste Sekretionsreiz für Insulin. Die Glucosemoleküle werden im Menschen über verschiedene Glucosetransporter insulinunabhängig von den β-Zellen aufgenommen und dort über Glykolyse, Citratzyklus und Atmungskette verstoffwechselt. Aus Tierversuchen mit Mäusen und Ratten ist bekannt, dass hier vornehmlich der Glukosetransporter 2 (GluT 2) für die Aufnahme verantwortlich ist. In menschlichen β-Zellen werden aber ebenso GluT 1 und GluT 3 exprimiert.[1]
In Folge der Glucoseverstoffwechslung steigt die ATP-Konzentration an und ATP-abhängige Kaliumkanäle verschließen sich. Es kommt folglich zu einer Depolarisation. Der Kalziumeinstrom durch spannungsabhängige Kalziumkanäle führt zur Einleitung der Exozytose. Dadurch fusionieren insulinhaltige Vesikel mit der Zellmembran. Durch die Entleerung des Vesikelinhaltes in den Extrazellulärraum kommt es zur Ausschüttung des Insulins aus den beta-Zellen.
Ein Konzentrationsanstieg von Fettsäuren und Aminosäuren übt ebenfalls einen schwachen fördernden Reiz auf die Insulinsekretion aus. Hormone wie Gastrin, Sekretin, GIP und GLP-1 wirken stimulierend auf die Insulinsynthese und -freisetzung, insbesondere beeinflussen sie die postprandiale Insulinausschüttung.
Allerdings wird auch in nüchternem Zustand Insulin ausgeschüttet, um den Blutglukoselevel konstant zu halten. Dieses Basalinsulin macht mehr als die Hälfte der täglichen Sekretion aus.
Die Insulinausschüttung erfolgt nicht linear, sondern oszillierend. Dabei wird alle 3 bis 6 Minuten Insulin in die Blutbahn abgegeben.
siehe auch: Insulinsekretion
Physiologie
Die Wirkung von Insulin wird über die Bindung an Insulinrezeptoren auf der Zelloberfläche des Leber-, Muskel- und Fettgewebes vermittelt, die in der Zelle eine intrazelluläre Signalkaskade, das sogenannte Insulinsignal auslösen. Insulin beeinflusst den Glucosestoffwechsel durch mehrere Mechanismen. Zu den wichtigsten biologischen Wirkungen gehören:
- Membraneffekte
- Beschleunigung der Glucoseaufnahme in Muskel- und Fettzellen (Translokation der Glucosetransporter)
- Beschleunigung der Aufnahme von Aminosäuren und Kalium in Muskel- und Fettzellen
- Beschleunigung der Aufnahme von Triglyceriden in Muskel- und Fettzellen
- Metabolische Effekte
- Induktion der Glykogensynthese und -speicherung in Leber und Muskel
- Steigerung der Triglyceridsynthese in Leber und Fettgewebe (sog. antilipolytische Wirkung)
- Speicherung von Aminosäuren im Muskel
- Hemmung der hepatischen Gluconeogenese
- Hemmung der Proteolyse
- Hemmung der Glykogenolyse
- Hemmung der Lipolyse
- Regulation des Zellwachstums und der Proliferation durch die Aktivierung der Transkription von Genen, die den Zellzyklus kontrollieren.
Aufgrund seiner muskelaufbauenden Eigenschaften wird Insulin im Sport auch als Anabolikum missbraucht.[2]
Plasma-Glukosespiegel und Insulinsekretion beeinflussen sich wechselseitig im Sinne eines Regelkreises. Dadurch kann der gesunde Organismus den Blutzuckerspiegel auch bei Störungen konstant halten. Die Leistung und Zuverlässigkeit der Kohlenhydrathomöostase wird dadurch erhöht, dass Insulin in antagonistischer Redundanz mit Gegenspieler-Hormonen wie Glukagon, Katecholaminen und Glukokortikoiden steht.
Labordiagnostik
Insulin wird labormedizinisch selten als Einzelwert bestimmt, sondern immer in Verbindung mit anderen Stoffwechselparametern, vor allem mit der Blutglukose.
Insulin ist in Vollblutproben instabil. Die Proben müssen sofort zentrifugiert und separiert werden, im Serum beträgt die Stabilität ca. 24 h. Bei längerer Probenlaufzeit muss das Serum eingefroren werden. Aus diesem Grund wird statt des Insulins häufig das C-Peptid bestimmt.
Die C-Peptid-Bestimmung erhöht die Aussagekraft der Insulinbestimmung oder ersetzt diese im Einzelfall. Gründe hierfür sind:
- Das C-Peptid wird in genau der gleichen Menge gebildet wie Insulin (Abspaltungsprodukt).
- Exogene Insulingaben stören die Bestimmung des C-Peptids nicht, die Insulinbestimmung jedoch schon.
- Endogene Insulin-Antikörper beinträchtigen die Messung von Insulin, nicht jedoch die des C-Peptids.
Bei Verdacht auf eine Hypoglycaemia factitia ist die gleichzeitige Insulin- und C-Peptid-Bestimmung sinnvoll.
Referenzwerte
| Bedingung | mU/l (µU/ml) | pmol/l |
|---|---|---|
| Nach 12 h Fasten | 6 - 25 | 36 - 150 |
| Nach 72 h Fasten | < 6 | < 36 |
| Nach maximaler Stimulation durch Glucose oder Glukagon | bis 200 | bis 1200 |
Die Messwerte sind methodenabhängig. Entscheidend ist der vom ausführenden Labor angegebene Referenzwert.
Interpretation
Einen erhöhten Insulinspiegel im Serum bezeichnet man als Hyperinsulinämie, erniedrigte Insulinwerte als Hypoinsulinämie.
Erhöhte Werte findet man bei
Erniedrigte Werte liegen vor bei
Typ 2-Diabetiker weisen meist eine kompensatorische Hyperinsulinämie (Insulinresistenz) auf, die mit einer Hyperglykämie einhergeht.
Insulinpräparate
Die Behandlung des Diabetes mellitus erfolgt heute grundsätzlich mit Humaninsulin, d.h. mit Insulinen, deren Aminosäuresequenz dem vom menschlichen Körper produzierten Insulin entspricht oder mit Insulinanaloga. Die früher verwendeten Tierinsuline (Schweineinsulin, Rinderinsulin) werden heute für Neueinstellungen nicht mehr eingesetzt. Bei Patienten, die allergisch auf gentechnisch produziertes Humaninsulin oder Insulinanaloga reagieren, wird Schweineinsulin weiterhin eingesetzt.
Die Einteilung der Insuline erfolgt nach ihrer Wirkdauer:
Kurz wirkende Insuline
Kurz wirkende Insuline werden bei Stoffwechselentgleisungen, zur Ersteinstellung, bei der intensivierten konventionellen Therapie (ICT), bei Verwendung einer Insulinpumpe und perioperativ eingesetzt (Bolusinsulin).
| Präparat | Initialwirkung | Wirkungsmaximum | Wirkungsdauer | |
|---|---|---|---|---|
Normalinsulin (Altinsulin)
|
s.c. | 30 min | 1 - 3 h | 5 - 8 h |
| i.v. | 5 - 7 min | 10 min | 20 min | |
Kurz wirkende Insulinanaloga
|
5 - 15 min | 1 h | 2 - 5 h | |
Verzögerungsinsuline
Verzögerungsinsuline werden eingeteilt in:
- Intermediärinsulin
- Langzeitinsulin: heute (2023) kaum noch in Verwendung, Ultratard® z.B. seit 2006 vom Markt
- Lang wirkende Insulinanaloga
- Ultralangzeitinsulin (Wocheninsulin)
Bei Intermediärinsulinen wird die Wirkung durch Verbindung des Insulins mit Protamin (Neutral Protamin Hagedorn, NPH-Insuline) verzögert. An Bedeutung verloren haben Verbindungen mit Surfen sowie die Verwendung von Proinsulin. In der Humanmedizin nicht mehr angewendet werden Insuline mit Zink (Lente-Insuline).
Verzögerungsinsuline dürfen nur subkutan injiziert werden. Sie werden u.a. zur Abdeckung des Basisinsulinbedarfs bei der ICT eingesetzt ("Basalinsuline").
| Gruppe | Präparate | Initialwirkung | Wirkungsmaximum | Wirkungsdauer |
|---|---|---|---|---|
| Intermediärinsuline | Huminsulin® basal | 45 - 90 min | 4 - 10 h | 10 - 20 h |
| Humulin® basal | ||||
| Insulatard® | ||||
| Insuman® basal | ||||
| Protaphane® | ||||
| Lang wirkende Insulinanaloga | Insulin detemir (Levemir®) | 2 - 3 h | 4 - 14 h | 20 - 24 h |
| Insulin glargin (Lantus®, Abasaglar®, Toujeo®) | 2 - 4 h | langes Plateau | ≥ 24 h | |
| Ultralangzeitinsuline | Insulin icodec (Awiqli®) | - | - | 1 Woche |
| Insulin Efsitora Alpha | - | - | 1 Woche |
Mischinsuline
Fixe Mischungen aus Normalinsulin und NPH-Insulinen werden zur 2 bis 3x täglichen Injektion im Rahmen der konventionellen Insulintherapie eingesetzt.
- Analog/NPH-Insuline (kein Spritz-Ess-Abstand - SEA)
- NovoMix® 30 und 70
- Humalog® Mix 25 und 50
- Normal/NPH-Insuline (Mischung: 50 % normal/50 % NPH)
- Mixtard® 50
- Normal/NPH-Insuline(Mischung: 30 % Normal/70 % NPH)
- Mixtard® 30
- Insuman® comb 25
- Lilly Profil III®
- Actraphane®
Darüber hinaus existieren viele andere Mischinsuline.
Verabreichung
Parenteral
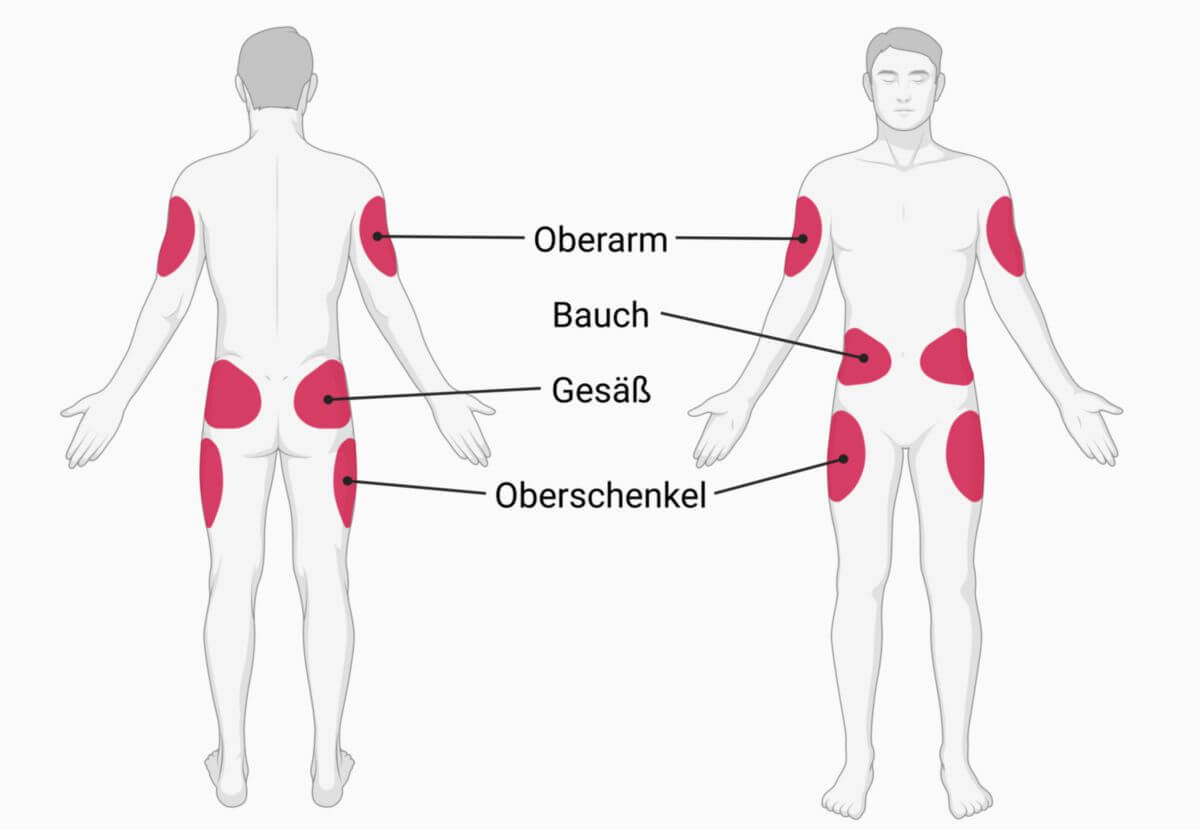
Insulin kann subkutan, intravenös oder (selten) intramuskulär appliziert werden. Die drei Applikationsarten besitzen eine sehr unterschiedliche Pharmakokinetik. Die Standardapplikation ist die subkutane Injektion, auf die sich auch die Angaben zum Wirkeintritt bzw. zur Wirkdauer beziehen. In den meisten Fällen wird dazu ein Insulinpen eingesetzt. Intravenös darf Insulin nur mit äußerster Vorsicht gespritzt werden (oder durch Infusor oder Pumpe, die Kleinstmengen abgibt), weil ein unmittelbarer Wirkungseintritt erfolgt.
Die intramuskuläre Verabreichung bringt meist eine Wirkungsbeschleunigung von ca. 30 bis 50%. Wenn jedoch in vernarbte Muskelareale gespritzt wird, kann die Wirkung sogar ganz ausbleiben.
Inhalativ
Eine weitere Darreichungsform stellt die Inhalation von Insulin mit anschließender Resorption über die Lungenkapillaren dar (siehe auch: inhalatives Insulin). Ein Nachteil der Methode ist, dass die Bioverfügbarkeit des Insulins nach Inhalation sehr unterschiedlich sein kann. Die Produktion des ersten inhalativen Insulins (Exubera®) wurde Ende 2007 vom Hersteller Pfizer eingestellt. 2014 wurde in den USA ein neues inhalatives Insulinpräparat zugelassen (Afrezza®).[3]
Oral
Ungeschützt wird oral gegebenes Insulin innerhalb von Minuten im oberen Gastrointestinaltrakt abgebaut. Deshalb ist bislang (2023) kein oral wirksames Insulin verfügbar. Es befinden sich jedoch neuere nanotechnologische Verfahren in der Entwicklung, mit denen das Problem der Deaktivierung ggf. gelöst werden kann. Ein Ansatz sind insulinbeladene Nanopartikel aus Chitosan.[4][5] Im Juni 2017 wurde eine Studie vorgestellt, die eine gleichwertige Wirkung des oral eingenommenen Insulins im Vergleich zu gespritzem zeigte. Aufgrund der schlechten Bioverfügbarkeit wird aber weiterhin an der Entwicklung eines Präparats geforscht.[6]
Bei tierexperimentellen Untersuchungen an diabetischen Ratten wurde Insulin in eine amphiphile Lipidstrukturen eingebettet und mit einer magensaftresistenten Kapsel appliziert. Es wurden sowohl schnell als auch langsam wirkende Insuline getestet. Bei einer entsprechenden Stärke der Beschichtung der Kapseln wurde eine Bioverfügbarkeit wie nach einer subkutanen Applikation erreicht.[7]
Wechselwirkungen
Wechselwirkungen bestehen mit Arzneimitteln, welche die Insulinausschüttung negativ beeinflussen oder zu Insulinresistenz führen können. Dazu zählen:
- Betablocker, Thiaziddiuretika: Hemmung der Insulinsekretion
- Antidepressiva (SSRI, MAO-Hemmer): Hemmung der Insulinsekretion
- Kortikosteroide: machen insulinabhängige Hemmung der hepatischen Glukosefreisetzung rückgängig, senken Insulinrezeptorbindungsaffinität
- Orale Kontrazeptiva: senken Insulinsensivität
Darüber hinaus treten Wechselwirkungen mit Medikamenten zur Behandlung des Typ-II-Diabetes auf, welche die Insulinsekretion steigern bzw. die Insulinresistenz verringern:
Überdosierung
Versehentlich oder missbräuchlich herbeigeführte erhöhte Spiegel von Insulin führen zu einer Hypoglykämie. Diese kann in schweren Fällen zu Bewusstlosigkeit, Koma oder dem Tod führen.
Literatur
- Laborlexikon.de; abgerufen am 29.03.2021
- Wilcox, G. Insulin and Insulin Resistance.The Clinical Biochemist Reviews.2005 May; 26(2):19-39.
Quellen
- ↑ Thorens B.GLUT2, glucose sensing and glucose homeostasis. Diabetologia. 2015 Feb;58(2):221-32. doi: 10.1007/s00125-014-3451-1.
- ↑ Andeson et al. Use of growth hormone, IGF-I, and insulin for anabolic purpose: Pharmacological basis, methods of detection, and adverse effects. Molecular and Cellular Endocrinology. 2018
- ↑ Deutsches Ärzteblatt - USA: Inhalatives Insulin zugelassen, abgerufen am 27.04.2023
- ↑ Fonte P, Araújo F, Reis S, Sarmento B. Oral insulin delivery: how far are we? J Diabetes Sci Technol. 2013 Mar 1;7(2):520-31.
- ↑ Li X, Qi J, Xie Y, Zhang X, Hu S, Xu Y, Lu Y, Wu W: coated with alginate/chitosan as oral insulin delivery systems: preparation, characterization, and hypoglycemic effect in rats. Int J Nanomedicine. 2013;8:23-32. doi: 10.2147/IJN.S38507.
- ↑ Studie: Daily, Long-Acting Oral Insulin Tablet Provides Comparable Glycemic Control to Insulin Glargine Injection in Patients with Type 2 Diabetes vorgestellt auf der American Diabetes Association's 77th Scientific Session, Juni 2017
- ↑ Strachan JB et al. A promising new oral delivery mode for insulin using lipid-filled enteric-coated capsules. Biomater Adv. 2023
siehe auch: Insulintherapie, Insulinanaloga, Insulinresistenz